Fluorescence is a relatively new character on the stage of science, but the history of fluorescence goes back 500 years! Even then, the property itself is not a man-made invention. Fluorescence is a naturally occurring feature of many minerals, plants, and even animals. Here we will see how the first observations of fluorescence slowly gave way to its now-ubiquitousness. Fluorescence is standard in natural science laboratories all over the world as the go-to tool for biological imaging.

Discovery of Fluorescence
Fluorescence is a property of many natural minerals and proteins present in living cells. Simply, the energy structure of the molecules allow them to absorb light, and then spit it back out a little different. The most well known is the glowing green jellyfish from which GFP (green-fluorescent protein, aptly named) is derived. But fluorescence actually got its name from Fluorite, a mineral that is many shades from green to purple. And, fluorite was already prized for its beauty in antiquity.
Pliny the Elder in the first century AD described the gemstone fluorite with the Latin terms “murrina” and “myrrhina” in Naturalis Historia. But, the name “fluorite” comes from the word meaning “flux” or “to flow.” Functionally this stone was used in extracting iron from ore to enhance the flow of the molten metals during the process. Pliny also noted objects carved from fluorite as prized among Romans.
First Origins of in the History of Fluorescence

The first inklings of noticing the glow of fluorescent molecules occurred as early as 1565. Bernardino de Sahagún. Sahagún was a Spanish Franciscan friar who, during his evangelical explorations, documented a lot of the existing culture and natural life in Mexico. He saw that the bark of the Eysenhardtia polystachya tree, known as “palo azul” (blue wood), had peculiar color properties.

This tree, Eysenhardtia polystachya, was also known as “kidney wood” due to its diuretic properties. Sahagún noted the “brightness” of infusions containing palo azul, in the Florentine Codex. Indeed, now we know palo azul contains matlaline, a known fluorophore (molecule with the ability to fluoresce). The Aztecs used this, among other things such as palygorskite clays, for a vibrant blue paint color resistant to fading, termed “maya blue.”
I tried to make Lignum Nephriticum and it turned out pretty good! OldyTimey fluorescent blue wood water. pic.twitter.com/jndcDVvK8E
— Evan Dumas (@eedumas) September 30, 2020

It would only be hundreds of years later that the phenomena leading to the brightness of Lignum nephriticum would be named and described.
The Naming and Study of Fluorescent Phenomena
In 1852, Bristish scientist Sir George Stokes first used the term “fluorescence” to describe the emission of light by the mineral fluorite (fluorspar) and uranium glass under illumination by ultraviolet (shortwave) light. You may also know this type of UV light as a “blacklight.”
Particularly, GG Stokes noticed that when these illuminated fluorescent samples emitted the absorbed UV light, they emitted light had a shorter wavelength. This observance indicated something in the molecules was modulating the incoming light.
“I am almost inclined to coin a word, and call the appearance fluorescence, from fluor-spar, as the analogous term opalescence is derived from the name of a mineral.”
G.G. Stokes, On the change of refrangibility of light, Philosophical Transactions of the Royal Society of London (1852) vol. 142, pp. 468, 479, 463–562, DOI: 10.1098/rstl.1852.0022ope.

In later characterizations of fluorescence, the shift in wavelength between the higher-energy absorbed and lower-energy emitted light came to be termed the “Stokes shift.”
Those who have taken Calculus III or Vector Calculus will also recognize the name from the infamous and highly useful “Stokes theorem” (yes, it’s the same guy.)
Though Stokes’ publication regarding fluorescence come out in 1852, legend has it that Lord Kelvin urged him to talk about it sooner. Apparently GG Stokes had talked of this discovery well before his formal documentation.(Thomson, William (2 February 1883). “The size of atoms”. 10: 185–213. ; see pp. 207–208)
Stokes Shift – The History of Fluorescence’s Biggest Contribution
During fluorescent light emission, the photons emitted have a longer wavelength than the light used to excite it. “Red-shifted” is the term for light going to a longer wavelength. The longest wavelength of visible light is red, while the shortest is blue.
The energy of a photon depends on its frequency, which is inverse to its wavelength. So, the longer wavelengths have lower energy, and the shorter have higher energy. Blue light is thus higher energy than red. The red-shifting characteristic thus indicates a loss of energy in the process of relaxation.

Where does the lost energy go, if not to light emission? This phase of decay is non-radiative emission for the very reason that it does not radiate light. The energy is sapped in a variety of other ways, but most often to the creation of phonons, which are in the category of quasiparticles since they act like particles but actually aren’t. Phonons are really just highly organized acoustic vibrations of the lattice medium. Therefore most of the time, the non-radiative energy lost during Stokes shift goes to lightly jiggling the lattice. Read more about what can happen when a crystal “moves” inside its lattice!
Further Characterizations in the History of Fluorescence
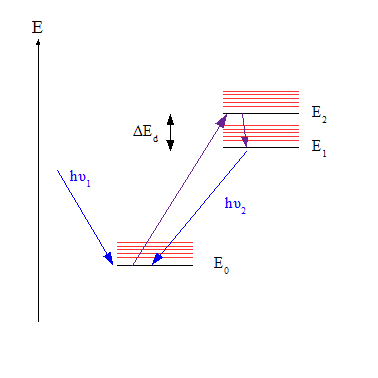
Russian physicist Aleksander Jabłoński is known to many modern-day students because he is the namesake of Jablonski diagrams. Jabonski diagrams are the foremost pictoral reference for describing fluorescence. Jablonski studied so in depth on the particulars photons go through to achieve the Stokes shift, that he created this useful diagram that students and researchers use today. He proposed this diagram in 1933 while working on his thesis.
In 1935, Jablonski gave a thesis On the influence of intermolecular interactions on the absorption and emission of light at the University of Warsaw. Jablonski focused his work on the “relaxation” that photons undergo between absorbing the incident light and emitting the modulated light.

As a element or molecule becomes excited upon absorbing incident light, the energy level jumps up, as shown by the blue arrow.
The red and green arrows depict the “relaxation” or “decay” stage which Jablonski studied most intimately. The red arrows show the transition which does not radiate light out. The green arrow, the last stage in relaxation (which, btw, lasts about a billionth of a second), is then the fluorescence, or emission of red-shift light occurs.
The Jablonski diagram gave a useful way for other researchers to characterize these energy shifts for particular cases. And these transitions happen on time scales so short our minds can’t really comprehend them.
Kasha’s Rule – The Final installment in the History of Fluorescence?
Michael Kasha was a Ukrainian-American (1920-2013) who studied at University of Michigan and UC Berkley before joining the faculty at Florida State University in 1951. Kasha studied molecular biophysics using spectroscopy. The use of fluorescent dyes to tag proteins in biological samples had been commonplace since some decades after fluorescences original characterization by Stokes in the 1930s.
It is of great interest to biology researchers in increase the photon count, or the intensity of the emitted light, to achieve a higher signal-to-noise ratio in experiments using fluorescent dyes. It seemed like, for simple molecules, that the wavelength (color) of the incident light did indeed determine the intensity emitted. But as Kasha studied more complex systems it began to seem like this wasn’t a fundamental property.
Kasha found out that the intensity emitted after the wavelength shift actually is not dependent on the wavelength of the incident light, at least not directly. Because the lowest energy states occur the most frequently in a system, these effects dominate many experiments.
Interestingly, acoustics in music also interested Kasha. He even invented and patented a new type of guitar that had enhanced acoustic sound, called the Kasha guitar. It looks a little funky, with the sound hole off center to the side of the strings, but evidently the sound is unparalleled amongst acoustic guitar designs.

The History of Fluorescence is Far From Over
With Kasha’s particular findings in the 20th century, many novel features awaiting characterization unlock. The history of fluorescence is still being written. Researchers now want to find out exceptions to his rule, which is mainly profound in the theoretical for now. But the implications of Kasha’s rule on the ability of energy states to dictate the level of radiative emission, is actually used in fields outside of fluorescence. These fields include photonics and nanoscience. Nanoparticles can give off higher luminosity by triggering the same effect. So biologists want to exploit this for even better imaging, in vivo and in real time!
Fluorescence Articles in the Pipeline
- ~~> How Fluorescence Works
- ~~> Mineral, Plant, and Animal Examples of Fluorescence
- ~~> Everyday Examples of Fluorescence
- ~~> Applications of Fluorescence
- ~~> Structure and Formation of Lignum nephriticum
- ~~> Fluorite Crystal’s structure and function
- ~~> Contributions of Sir GG Stokes
Let me know in the comments what you’re looking forward to learning about next!



Pingback: 10 Fluorescence Examples in Daily Life to Explore Light - Abnormal Ways
Pingback: How Fluorescence Works Step by Step Easily Explained
Pingback: Examples of Fluorescence in Nature That Will Brighten Your Mind - Abnormal Ways
Pingback: 10 Types of Luminescence (including rare and overlapping type